Answer:
A = 65.46 u
Step-by-step explanation:
Given that,
The composition of zinc is as follows :
Zn-64 = 48.63%
Zn-66 = 27.90%
Zn-67 = 4.10%
Zn-68 = 18.75%
Zn-70 = .62%
We need to find the average atomic mass of the given element. It can be solved as follows :
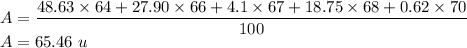
So, the average atomic mass of zinc is 65.46 u.