Answer: The mass density is 1166.36
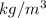 .
.
Step-by-step explanation:
Given: Weight of sample in air
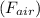 = 500 N
= 500 N
Weight of sample in alcohol
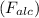 = 200 N
= 200 N
Specific gravity = 0.7 =
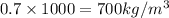
Formula used to calculate Buoyant force is as follows.
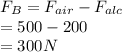
Hence, volume of the material is calculated as follows.
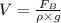
where,
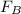 = Buoyant force
= Buoyant force
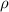 = specific gravity
= specific gravity
g = acceleration due to gravity = 9.81
Substitute the values into above formula.
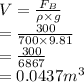
Now, mass of the material is calculated as follows.
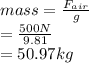
Therefore, density of the material or mass density is as follows.
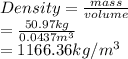
Thus, we can conclude that the mass density is 1166.36
 .
.