Answer:
The Experimental Molar Volume in L/mol of the Hydrogen gas=23.36L/mol
Step-by-step explanation:
We are given that
Volume of H2 at STP=52.8mL
Mass of magnesium metal ,M(Mg)=0.055g
We have to find the Experimental Molar Volume in L/mol of the Hydrogen gas.
Molar mass of Mg=24.305 g/mol
Number of moles=
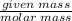
Using the formula
Number of moles of Mg=
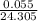 moles
moles
Number of moles of Mg=0.00226moles
Number of moles of Mg=Number of moles of H2
Number of moles of H2=0.00226moles
Molar volume of Hydrogen gas (H2)=
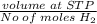
Molar volume of Hydrogen gas (H2)=
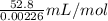
Molar volume of Hydrogen gas (H2)=
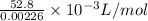
Molar volume of Hydrogen gas (H2)=23.36L/mol
Hence, the Experimental Molar Volume in L/mol of the Hydrogen gas=23.36L/mol