WHAT IS KNOWN AS HYBRIDIZATION
It is the change in the orbitals of the central atom of the molecule to form bonds with other atom if same type or another type.
The hybrid orbitals r of same level .
The Hybridization takes place in between orbitals of equal or very less energy levels to form same level of energy in all orbitals.
The orbitals combine is always equal to The number of orbitals formed
The names of hybridized orbitals r kept according to the orbital which r combined to form them
Eg:-
sp orbital :- one s and one p combine to form sp orbital
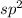 orbital :- one s and two p orbitals combine to form it..
orbital :- one s and two p orbitals combine to form it..