Answer:
In a chemical reaction, equilibrium is achieved when the forward and reverse reactions occur at the same rate.
Equilibrium is influenced by the following:
1) pressure
2) temperature
3) concentration
Bear in mind that CATALYST HAS NO IMPACT ON THE EQUILIBRIUM; IT ONLY INCREASES THE RATE.
PRESSURE:
Only if the reaction's left side contains a greater number of molecules can the pressure be increased in order to boost yield.
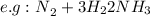
Thus, because the left side has four molecules and the right side has two, you should increase pressure to increase yield.
TEMPERATURE:
In order to increase yield, raise the temperature only in ENDOTHERMIC FORWARD reactions.
CONCERNTRATION:
Removing the formed product increases yield.
I hope this helps you
:)