Answer:
The sign of work will be negative.
Step-by-step explanation:
The work of the system is given by:
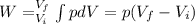
Where:
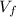 : is the final volume = 31 L
: is the final volume = 31 L
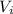 : is the initial volume = 63 L
: is the initial volume = 63 L
p: is the pressure
Even though we do not have the pressure value, by knowing the values of the initial and final volume we can predict the sign of work.
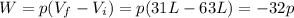
Therefore, the sign of work will be negative.
I hope it helps you!