Answer: At equilibrium, the partial pressure of
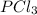 is 0.0330 atm.
is 0.0330 atm.
Step-by-step explanation:
The partial pressure of
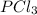 is equal to the partial pressure of
is equal to the partial pressure of
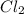 . Hence, let us assume that x quantity of
. Hence, let us assume that x quantity of
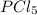 is decomposed and gives x quantity of
is decomposed and gives x quantity of
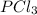 and x quantity of
and x quantity of
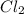 .
.
Therefore, at equilibrium the species along with their partial pressures are as follows.
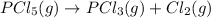
At equilibrium: 0.123-x x x
Now, expression for
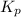 of this reaction is as follows.
of this reaction is as follows.
![K_(p) = ([PCl_(3)][Cl_(2)])/([PCl_(5)])\\0.0121 = (x * x)/((0.123 - x))\\x = 0.0330](https://img.qammunity.org/2022/formulas/chemistry/college/e0sgyurx2i1reypmkw4cwwnvogfowx0yxo.png)
Thus, we can conclude that at equilibrium, the partial pressure of
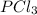 is 0.0330 atm.
is 0.0330 atm.