Answer:
To determine the enthalpy and entropy of dissolving a compound, you need to measure the Ksp at multiple temperatures. Then, plot ln(Ksp) vs. 1/T. The slope of the plotted line relates to the enthalpy (ΔH) of dissolving and the intercept of the plotted line relates to the entropy (ΔS) of dissolving.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us use the thermodynamic definition of the Gibbs free energy and its relationship with Ksp as follows:
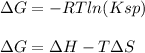
Thus, by combining them, we obtain:
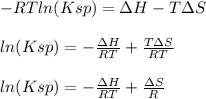
Which is related to the general line equation:
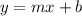
Whereas:
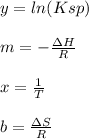
It means that we answer to the blanks as follows:
To determine the enthalpy and entropy of dissolving a compound, you need to measure the Ksp at multiple temperatures. Then, plot ln(Ksp) vs. 1/T. The slope of the plotted line relates to the enthalpy (ΔH) of dissolving and the intercept of the plotted line relates to the entropy (ΔS) of dissolving.
Regards!