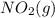Answer: The correct option is
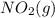
Step-by-step explanation:
A catalyst is defined as the chemical species that increases the reaction rate but does not participate in it and is left behind after the completion.
A homogeneous catalyst is one that is present in the same phase as the reactants and products.
A heterogeneous catalyst is one that is present in different phase as that of reactants and products.
For the given chemical reaction:
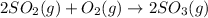
As all the reactants and products are in gaseous state so, the homogeneous catalyst must also be in the gaseous state only.
Hence, the correct option is