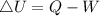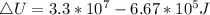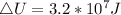Answer:
Step-by-step explanation:
From the question we are told that:
Energy
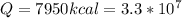
Initial Volume
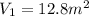
Final Volume
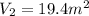
a)
Generally the equation for Work done is mathematically given by
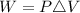
Where
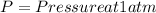
Therefore
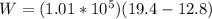
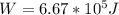
a)
Generally the equation for Change in internal energy of the gas is mathematically given by