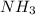Answer: The equilibrium will shift in the direction of
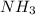
Step-by-step explanation:
The chemical equation for the aqueous solution of ammonia follows:
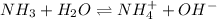
According to Le-chtelier's principle:
If there is any change in the variables of the reaction, then the equilibrium will shift in that direction of equilibrium to minimize the effect.
If we add more amount of
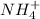 to the solution, more of the products will be present. But according to Le-chtelier's principle, to minimize this effect, the equilibrium will shift in the backward direction that in the direction of
to the solution, more of the products will be present. But according to Le-chtelier's principle, to minimize this effect, the equilibrium will shift in the backward direction that in the direction of
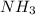
Hence, the equilibrium will shift in the direction of