Answer: The pH of the solution is 11.24
Step-by-step explanation:
We are given:
Molarity of ammonia = 0.2 M
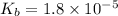
The given chemical equation follows:
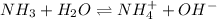
I: 0.2
C: -x +x +x
E: 0.2-x x x
The expression for equilibrium constant follows:
![K_b=([NH_4^+][OH^-])/([NH_3])](https://img.qammunity.org/2022/formulas/chemistry/college/xdfor96symsa9o515d7xhdjmg4jvzsmhms.png)
Putting values in above expression, we get:
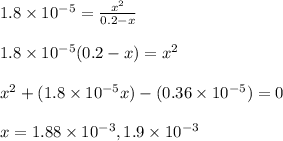
Neglecting the negative value of x as concentration cannot be negative.
So,
![[OH^-]=x=1.88* 10^(-3)M](https://img.qammunity.org/2022/formulas/chemistry/college/uolhvclxxh4dwtf2frejk3p81e6ozjocr9.png)
pOH is defined as the negative logarithm of hydroxide ion concentration present in the solution.
![pOH=-\log [OH^-]](https://img.qammunity.org/2022/formulas/chemistry/high-school/eow64ghspz91qh8x39ozfikdhng1azfkyv.png)
Putting values in above equation, we get:
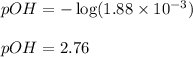
We know:
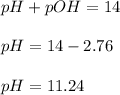
Hence, the pH of the solution is 11.24