Answer:
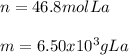
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to calculate both moles and grams of lanthanum by using the Avogadro's number as a relationship of atoms to moles and its atomic mass as a relationship to moles to grams to obtain the following:
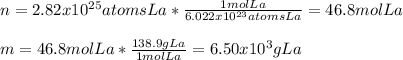
Regards!