Answer: The volume of the gas is 178.76 L
Step-by-step explanation:
The ideal gas equation is given as:
.......(1)
where
P = pressure of the gas = 675 torr
V = volume of gas = ?
n = number of moles of gas = 6.45 moles
R = Gas constant = 62.36 L.torr/mol.K
T = temperature of the gas =
![27^oC=[27+273]K=300K](https://img.qammunity.org/2022/formulas/chemistry/college/eistx1ciz2e3omo8k4hcoiuiq2dclhqupy.png)
Putting values in equation 1, we get:
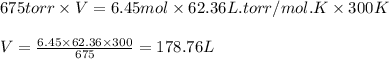
Hence, the volume of the gas is 178.76 L