Answer:
810.93
Explanation:
Let the pressure be given by P and the volume be V.
Since pressure is inversely proportional to volume, we can write;
P ∝
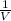
=> P =
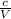 -------------(i)
-------------(i)
Where;
c = constant of proportionality.
When the volume of the gas is 2 cubic feet, pressure is 1000 pounds per square foot.
V = 2 ft³
P = 1000lb/ft²
Substitute these values into equation (i) as follows;
1000 =
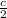
=> c = 2 x 1000
=> c = 2000 lbft
Substituting this value of c back into equation (i) gives
P =
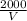
This is the general equation for the relation between the pressure and the volume of the given gas.
To calculate the work done W by the gas, we use the formula
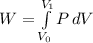
Where;
V₁ = final volume of the gas = 3ft³
V₀ = initial volume of the gas = 2ft³
Substitute P =
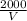 , V₁ = 3ft³ and V₀ = 2ft³
, V₁ = 3ft³ and V₀ = 2ft³
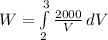
Integrate
W = 2000ln[V]³₂
W = 2000(In[3] - ln[2])
W = 2000(0.405465108)
W = 810.93016
W = 810.93 [to 2 decimal places]
Therefore, the work done by the gas for the given pressure and volume is 810.93