Answer:
Isotopy
Step-by-step explanation:
Isotopes are various forms of atoms of the same element with varying numbers of neutrons. Although all isotopes of an element possess the same atomic number as well as the number of protons, but their atomic weights differ. Chemically, isotopes of the same element have identical characteristics, but nuclear properties differ.
From the given information;
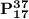 and
and
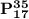 are called isotopes and the phenomena exhibited between is known as (isotopy)
are called isotopes and the phenomena exhibited between is known as (isotopy)