Answer: The structures of all the acid derivatives are attached below.
Step-by-step explanation:
Some of the acid derivatives are:
- Esters
- Amides
- Acid anhydrides
- Acid halides
To name a compound, first look for the longest possible carbon chain.
Amide group is a type of functional group where an amine group is attached to a carbonyl group. The general formula of amide is
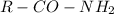 , where R is an alkyl or aryl group.
, where R is an alkyl or aryl group.
Esters are a kind of organic molecules having functional groups, where R and R' are the alkyl or aryl groups. They are formed by the combination of alcohol and carboxylic acid.
These functional group compounds are named in two words which is alkyl alkanoates, where alkyl refers to the alcoholic part and alkanoate refers to the carboxylic acid part of the molecule. The numbering of the parent chain in esters is done from the carboxylic carbon. The alkyl part is not given any numbers.
For (a): Ethyl 2-hydroxypropanoate
The longest possible carbon chain has 3 carbon atoms and prefix used will be 'prop-'.
A hydroxy (-OH) group is attached to the 2nd position.
The structure is attached in the image below.
For (b): Butyl 3,3-dimethyhexanoate
The longest possible carbon chain has 6 carbon atoms and prefix used will be 'hex-'.
2 methyl groups are attached at the 3rd position
The structure is attached in the image below.
For (c): N-Ethyl-N-methylbenzamide
The longest possible carbon chain is a benzene ring
1 methyl and 1 ethyl groups are attached directly to the N-atom
The structure is attached in the image below.
For (d): 2,3-Dibromohexanamide
The longest possible carbon chain has 6 carbon atoms and prefix used will be 'hex-'.
2 bromo groups are attached at the 2nd and 3rd position
The structure is attached in the image below.