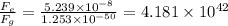Answer:
The ratio of the electrostatic force to the gravitational force between the electrons is 4.181 x 10⁴²
Step-by-step explanation:
Given;
charge of electron, Q = 1.602 x 10⁻¹⁹ C
mass of an electron, m = 9.1 x 10⁻³¹ kg
distance between the two electrons, r = 6.64 x 10⁻¹¹ m
The electrostatic force between the electrons is calculated using Coulomb's law;
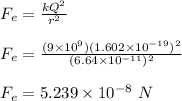
The gravitaional force between the electrons is calculated as;
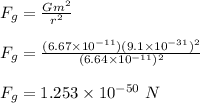
The ratio of the electrostatic force to the gravitational force between the electrons;