Answer:
Analysis of a sample of a compound composed of carbon, hydrogen, and oxygen shows that the sample contains 18.80 g of C, 2.367 g of H, and 25.04 g of O.
The properties of the compound suggest that the molar mass should be 59.04 g/mol.
How many carbon atoms are there in one molecule of the compound?
Step-by-step explanation:
Empirical formula mass is
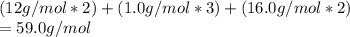
Empirical formula mass =molecular formula mass
Hence,
empirical formula is same as molecular formula.
That is ---
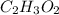 .
.
So, the molecule has two carbon atoms in one molecule of the compound.