Answer:
His results will be skewed because there was more water than stock solution. Which would cause the percentage solution to be less than 50% therefore the density would be less than the actual value.
Step-by-step explanation:
The solution will have percentage less than that of 50%. Therefore the density would be less than the actual value.
Suppose there should be 50 mL of the solution, and he added 60 mL. So 10 mL of the solution is added more.
Suppose the mass of the solute is m.
Originally, the density is =
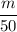
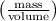
Now after adding extra 10 mL , the density becomes
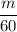 .
.
Therefore,
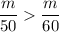
So the density decreases when we add more solution.