Answer:
Option B
Step-by-step explanation:
From the question we are told that:
Equation
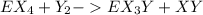
Bond enthalpies are:
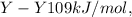
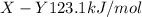
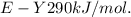
Generally the equation for the energy used to break E-X and Y-Y is mathematically given by
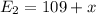
Therefore
Total Energy Liberated is
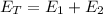
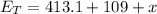
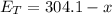
Since
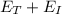
Therefore
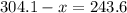
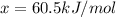
Option B