Answer:
The volume of CO₂ at STP is equal to 13.73 L.
Step-by-step explanation:
Given mass, m = 27 g
The molar mass of CO₂ = 44 g/mol
Let n is the number of moles. So,
n = given mass/molar mass
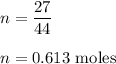
Let V is the volume of CO₂. So,
V = n×V'
Where
V' is the molar volume = 22.4 L
So,
V = 0.613 × 22.4
V = 13.73 L
Hence, the volume of CO₂ at STP is equal to 13.73 L.