Answer: The degree of ionization of a weak acid is greater than 50% but less than 100%.
Step-by-step explanation:
An acid which dissociates partially or weakly when dissolved in water is called a weak acid.
For example, acetic acid
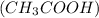 is a weak acid.
is a weak acid.
Hence, the degree of ionization of a weak acid is greater than 50% but less than 100%.
Thus, we can conclude that the degree of ionization of a weak acid is greater than 50% but less than 100%.