Answer:
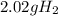
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to to calculate the mass of hydrogen gas by firstly setting up the undergoing chemical reaction:
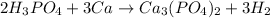
Thus, we apply the following stoichiometric setup, whereas the atomic mass of calcium is 40.08 g/mol, that of hydrogen is 2.02 g/mol and the mole ratio of these two substances is 3:3:
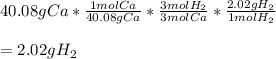
Regards!