Answer:
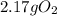
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to calculate the mass of 4.09 x 10^22 molecules of oxygen gas by firstly keeping in mind that that 1 mole of it has a mass of 32.0 g and secondly that 1 mole of any substance contains 6.022x10^23 representative units, in this case, molecules of O2, and thus, the appropriate setup to perform this conversion is shown below:
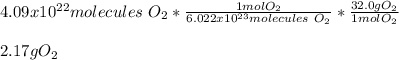
Regards!