Answer:
Approximately
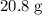 .
.
Step-by-step explanation:
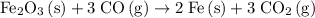 .
.
Relative atomic mass:
Formula mass:
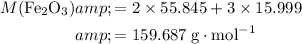 .
.
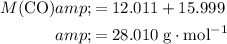 .
.
Number of moles of
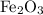 formula units in
formula units in
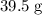 of this compound:
of this compound:
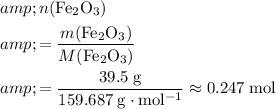 .
.
Refer to the balanced equation for this reaction.
- Coefficient of
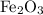 :
:
 .
. - Coefficient of
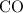 :
:
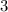 .
.
Hence, for every formula unit of
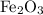 that this reaction consumes,
that this reaction consumes,
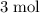 of
of
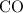 molecules would also need to be consumed. Therefore, if neither reactant is in excess:
molecules would also need to be consumed. Therefore, if neither reactant is in excess:
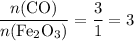 .
.
Calculate the number of moles of
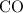 required to react with that
required to react with that
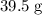 of
of
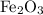 :
:
![\begin{aligned}&n({\rm CO}) \\ &= n({\rm Fe_2O_3}) \cdot \frac{n({\rm CO})}{n({\rm Fe_2O_3})} \\[0.5em] &\approx 0.247\; \rm mol * 3 \approx 0.742\; \rm mol\end{aligned}](https://img.qammunity.org/2022/formulas/chemistry/college/4ti5qgia8o4xhae74qnjfuz676k7k1i4de.png) .
.
Make use of the formula mass of
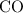 to find the mass of that
to find the mass of that
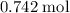 of
of
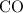 molecules:
molecules:
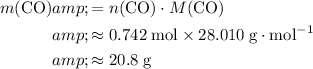 .
.