Answer:
If we look at the ground state (electrons in the energetically lowest available orbital) of oxygen, the electron configuration is
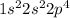 . If the element were to become excited, the electron could occupy an infinite number of orbitals. However, in most texts, the example will be the next available one. So for oxygen, it might look like this:
. If the element were to become excited, the electron could occupy an infinite number of orbitals. However, in most texts, the example will be the next available one. So for oxygen, it might look like this:
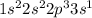 - where the valence electron now occupies the 3s orbital in an excited (i.e. not ground) state.
- where the valence electron now occupies the 3s orbital in an excited (i.e. not ground) state.
So, the electron configuration of oxygen in its excited state is
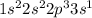 .
.