Answer:
The volume of the helium balloon is 0.306 L.
Step-by-step explanation:
Boyle's law states that the volume occupied by a given gaseous mass at constant temperature is inversely proportional to pressure. That is, if the pressure increases, the volume decreases, while if the pressure decreases, the volume increases.
Mathematically, Boyle's law indicates that the product of pressure and volume always has the same value:
P*V= k
Assuming that you have a certain volume of gas V1 that is at a pressure P1 at the beginning, by varying the volume of gas to a new value V2, then the pressure will change to P2, and it will be fulfilled:
P1*V1= P2*V2
In this case:
- P1= 2 atm
- V1= 0.15 L
- P2= 0.97 atm
- V2= ?
Replacing:
2 atm* 0.15 L= 0.97 atm* V2
Solving:
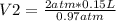
V2= 0.306 L
The volume of the helium balloon is 0.306 L.