Answer:
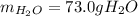
Step-by-step explanation:
Hello there!
In this case, since the formation of water from hydrogen and oxygen is:
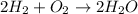
Whereas we find a 2:2 mole ratio of hydrogen to water. In such a way, by using the Avogadro's number, the aforementioned mole ratio and the molar mass of water (18.02 g/mol), we obtain the following grams of water product:
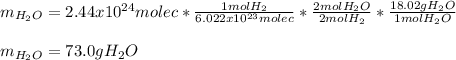
Regards!