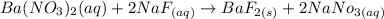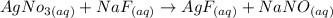Answer:
sodium fluoride
Step-by-step explanation:
When we add sodium fluoride to a solution of a given mixture, we get barium fluoride from Ba, which would be an insoluble salt, and silver fluoride from Ag, which would be a soluble salt.
The solubility rule will be used to determine the barium salt that forms as a precipitate and leaves Ag+ salt in the solution.
From the following equations, we will see that the precipitate is formed in Ba but Ag remains dissolved in the solution.