Answer: The half-reactions represents reduction are as follows.
Step-by-step explanation:
A half-reaction where addition of electrons take place or a reaction where decrease in oxidation state of an element takes place is called reduction-half reaction.
For example, the oxidation state of Cr in
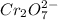 is +6 which is getting converted into +3, that is, decrease in oxidation state is taking place as follows.
is +6 which is getting converted into +3, that is, decrease in oxidation state is taking place as follows.
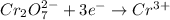
Similarly, oxidation state of Mn in
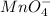 is +7 which is getting converted into +2, that is, decrease in oxidation state is taking place as follows.
is +7 which is getting converted into +2, that is, decrease in oxidation state is taking place as follows.
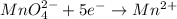
Thus, we can conclude that half-reactions represents reduction are as follows.