Answer:
The estimated atomic of silicon is 28.082 a.m.u.
Step-by-step explanation:
The estimated atomic mass of silicon (
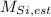 ), in a.m.u., is equal to the weighted average of atomic masses of silicon available in nature and in terms of their abundances:
), in a.m.u., is equal to the weighted average of atomic masses of silicon available in nature and in terms of their abundances:
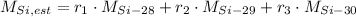 (1)
(1)
Where:
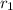 ,
,
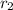 ,
,
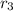 - Relative abundance of each isotope, no unit.
- Relative abundance of each isotope, no unit.
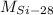 ,
,
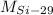 ,
,
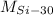 - Atomic mass of each isotope, in a.m.u.
- Atomic mass of each isotope, in a.m.u.
If we know that
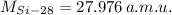 ,
,
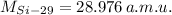 ,
,
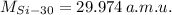 ,
,
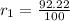 ,
,
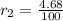 and
and
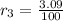 , then the estimated atomic mass of silicon is:
, then the estimated atomic mass of silicon is:
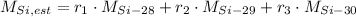
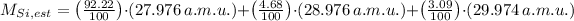
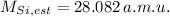
The estimated atomic of silicon is 28.082 a.m.u.