Answer: Molarity when 25.0 g of the compound
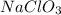 is placed in 85.0 mL of solution is 294.12 M.
is placed in 85.0 mL of solution is 294.12 M.
Step-by-step explanation:
Given: Mass = 25.0 g
Volume = 85.0 mL (1 mL = 0.001 L) = 0.085 L
Molarity is the number of moles of a substance divided by volume in liter.
Hence, molarity of given solution is calculated as follows.
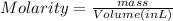
Substitute the values into above formula as follows.
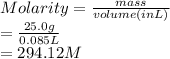
Thus, we can conclude that molarity when 25.0 g of the compound
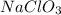 is placed in 85.0 mL of solution is 294.12 M.
is placed in 85.0 mL of solution is 294.12 M.