Answer:
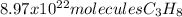
Step-by-step explanation:
Hello there!
In this case, according to the given chemical reaction and the fact that 22.4 L of a gas are occupied by 1 mol at standard pressure and temperature conditions, it will be possible for us to calculate the number of molecules of propane, by using the Avogadro's number, the 1:3 mole ratio with carbon dioxide and the aforementioned volume-mole ratio to obtain:
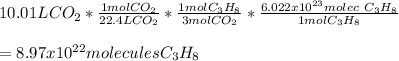
Regards!