Answer: The value of
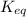 for this reaction is 1.578.
for this reaction is 1.578.
Step-by-step explanation:
Given: Initial moles of
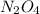 = 0.4 mol
= 0.4 mol
Volume = 1.00 L
Therefore, initial concentration of
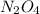 is calculated as follows.
is calculated as follows.
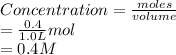
Now, ICE table for the given reaction equation is as follows.
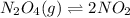
Initial: 0.4 0
Change: -x +2x
Equib: 0.4 - x = 0.0055 2x
Hence, the value of x is calculated as follows.
0.4 - x = 0.0055
x = 0.4 - 0.0055
= 0.3945
Now, the
![[NO_(2)]](https://img.qammunity.org/2022/formulas/chemistry/college/9ldqoeyboxa4myc6rvt5tmrcf9a087tszc.png) is calculated as follows.
is calculated as follows.
2x =
![[NO_(2)]](https://img.qammunity.org/2022/formulas/chemistry/college/9ldqoeyboxa4myc6rvt5tmrcf9a087tszc.png) =
=
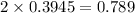
Therefore,
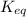 for the given reaction is calculated as follows.
for the given reaction is calculated as follows.
![K_(eq) = ([NO_(2)]^(2))/([N_(2)O_(4)])\\= ((0.789)^(2))/((0.3945))\\= 1.578](https://img.qammunity.org/2022/formulas/chemistry/college/6bu5dspllqy5nqir9gedwzcscylhnq5ip0.png)
Thus, we can conclude that
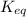 for this reaction is 1.578.
for this reaction is 1.578.