Answer:
Q = 360 Joules
Step-by-step explanation:
specific heat of copper = 0.400 J/g° C
mass of copper = 30 g
initial temperature = 20.0°C
final temperature = 50.0°C
Using the formula:
Q = mcΔT
where;
Q = Heat Energy
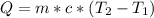
Q = (30 × 0.400)(50-20)
Q = 360 Joules