Answer:
pH = 13.1
Step-by-step explanation:
Hello there!
In this case, according to the given information, we can set up the following equation:

Thus, since there is 1:1 mole ratio of HCl to KOH, we can find the reacting moles as follows:
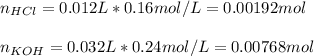
Thus, since there are less moles of HCl, we calculate the remaining moles of KOH as follows:
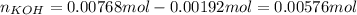
And the resulting concentration of KOH and OH ions as this is a strong base:
![[KOH]=[OH^-]=(0.00576mol)/(0.012L+0.032L)=0.131M](https://img.qammunity.org/2022/formulas/chemistry/college/3lrphpgungxsh99swrhcwjcixph5gp88je.png)
And the resulting pH is:
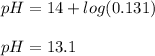
Regards!