Answer:
18.6 ml
Step-by-step explanation:
From the balanced equation given:
Volume of oxygen = 27.9 mL
From the equation, we will see that three moles of oxygen react with CS_2 to give two moles of sulfur dioxide (SO_2)
Also, three mL of O_2 yields two mL of SO_2
Now, to calculate the volume of SO_2; we have:
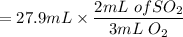
= 18.6 mL of SO_2