Answer:
1. C = 0.73 M.
2. pH = 0.14
Step-by-step explanation:
The reaction is the following:
HCl + NH₃ ⇄ NH₄⁺Cl⁻
From the titration, we can find the number of moles of HCl that were neutralized by the ammonia.
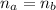
Where "a" is for acid and "b" is for base.
The number of moles is:

Where "C" is for concentration and "V" for volume.
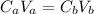
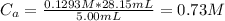
Hence the initial concentration of the acid is 0.73 M.
The original pH of the acid is given by:
![pH = -log([H^(+)])](https://img.qammunity.org/2022/formulas/chemistry/college/nd7uu1zdiir7bsf3i9tpwhne2qb6y4cfjb.png)
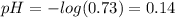
Therefore, the original pH of the acid is 0.14.
I hope it helps you!