Answer:
n = 1.56 x 10¹⁷ electrons
Step-by-step explanation:
First of all, we will calculate the current passing through wire:
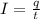
where,
I = current = ?
q = charge = 9 mC = 0.009 C
t = time = 3.6 s
Therefore,
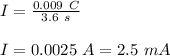
Now, for the same current in 10 s time the charge will be:
q = It = (0.0025 A)(10 s)
q = 0.025 C
Now, the number of electrons can be given as:
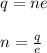
where,
n = no. of electrons = ?
q = charge = 0.025 C
e = charge on single electron = 1.6 x 10⁻¹⁹ C
Therefore,
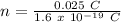
n = 1.56 x 10¹⁷ electrons