Answer:
sample B contains the larger density
Step-by-step explanation:
Given;
volume of sample A, V = 300 mL = 0.3 L
Molarity of sample A, C = 1 M
volume of sample B, V = 145 mL = 0.145 L
Molarity of sample B, C = 1.5 M
molecular mass of sodium chloride, Nacl = 23 + 35.5 = 58.5 g/mol
Molarity is given as;
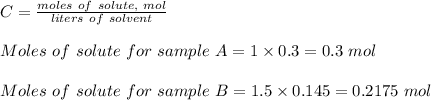
The reacting mass for sample A = 0.3mol x 58.5 g/mol = 17.55 g
The reacting mass for sample B = 0.2175 mol x 58.5 g/mol = 12.72 g
The density of sample A
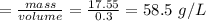
The density of sample B
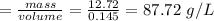
Therefore, sample B contains the larger density