Answer:
Reaction will move rightwards.
Step-by-step explanation:
Hello there!
In this case, according to the proposed question, it turns out possible for us to figure out the effect of the addition of silver nitrate, by using the following representative equation for the formation of the silver chloride precipitate:
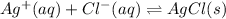
Now, due to the addition of silver nitrate, we will be actually adding silver ions to the solution, which means that, in terms of the Le Ch atelier's principle, the reaction will shift to the right towards the formation of more silver chloride precipitate.
Best regards!