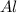Answer:
"2.48 mole" of H₂ are formed. A further explanation is provided below.
Step-by-step explanation:
The given values are:
Mole of Al,
= 3.22 mole
Mole of HBr,
= 4.96 mole
Now,
(a)
The number of mole of H₂ are:
⇒
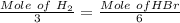
or,
⇒
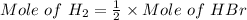
⇒
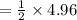
⇒
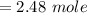
(b)
The limiting reactant is:
=
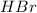
(c)
The excess reactant is:
=