Answer: If the oxidation number of copper changes from 0 to +2 then yes this is a redox reaction as copper lost 2 electrons and was oxidized.
Step-by-step explanation:
When an element or atoms loses an electron then it acquires a positive charge.
This charge on an atom represents its oxidation state.
For example,
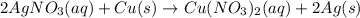
Here, oxidation number of copper changes from 0 to +2.
A redox reaction in which electrons are removed from an atom or a reaction where an increase in oxidation number of an atom takes place is called oxidation-half reaction. The atom itself gets oxidized.
This means that here copper lost 2 electrons and was oxidized.
Thus, we can conclude that if the oxidation number of copper changes from 0 to +2 then yes this is a redox reaction as copper lost 2 electrons and was oxidized.