Answer:
the concentration of free Ca2⁺ in this solution is 7.559 × 10⁻⁷
Step-by-step explanation:
Given the data in the question;
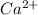 +
+
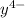 ⇄
⇄
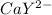
Formation constant Kf
Kf =
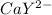 / ( [
/ ( [
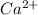 ][
][
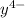 ] ) = 5.0 × 10¹⁰
] ) = 5.0 × 10¹⁰
Now,
[
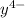 ] =
] =
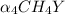 ; ∝₄ = 0.35
; ∝₄ = 0.35
so the equilibrium is;
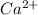 +
+
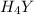 ⇄
⇄
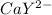 + 4H⁺
+ 4H⁺
Given that;
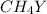 =
=
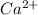 { 1 mol
{ 1 mol
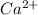 reacts with 1 mol
reacts with 1 mol
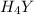 }
}
so at equilibrium,
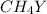 =
=
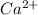 = x
= x
∴
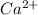 +
+
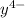 ⇄
⇄
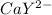
x + x 0.010-x
since Kf is high, them x will be small so, 0.010-x is approximately 0.010
so;
Kf =
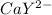 / ( [
/ ( [
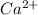 ][
][
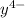 ] ) =
] ) =
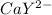 / ( [
/ ( [
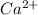 ][
][
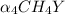 ] ) = 5.0 × 10¹⁰
] ) = 5.0 × 10¹⁰
⇒
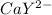 / ( [
/ ( [
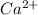 ][
][
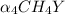 ] ) = 5.0 × 10¹⁰
] ) = 5.0 × 10¹⁰
⇒ 0.010 / ( [x][ 0.35 × x] ) = 5.0 × 10¹⁰
⇒ 0.010 / 0.35x² = 5.0 × 10¹⁰
⇒ x² = 0.010 / ( 0.35 × 5.0 × 10¹⁰ )
⇒ x² = 0.010 / 1.75 × 10¹⁰
⇒ x² = 0.010 / 1.75 × 10¹⁰
⇒ x² = 5.7142857 × 10⁻¹³
⇒ x = √(5.7142857 × 10⁻¹³)
⇒ x = 7.559 × 10⁻⁷
Therefore, the concentration of free Ca2⁺ in this solution is 7.559 × 10⁻⁷