Answer: A volume of 2997 liters of fluorine gas is needed to form 999 L of sulfur hexafluoride gas if the given reaction takes place at 2.00 atm and 273.15 K.
Step-by-step explanation:
The given reaction is as follows.
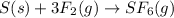
This show that 3 moles of fluorine is reacting to give 1 moles of sulfur hexafluoride.
According to the ideal gas formula,
PV = nRT
This means that volume of a gas is directly proportional to the number of moles. Hence, volume of fluorine required is calculated as follows.
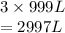
Thus, we can conclude that a volume of 2997 liters of fluorine gas is needed to form 999 L of sulfur hexafluoride gas if the given reaction takes place at 2.00 atm and 273.15 K.