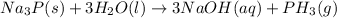Answer:
The formula of the gas produced is
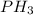 , and the balanced equation is :
, and the balanced equation is :
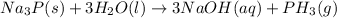
Step-by-step explanation:
The chemical reaction between lithium nitride and water gives lithium hydroxide and ammonia gas. The chemical equation of this reaction will be given as:
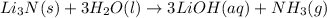
And when sodium phosphide reacts with water is gives an aqueous solution of sodium hydroxide and hydrogen phosphide gas also called phosphine gas.
The chemical equation of this reaction will be given as:
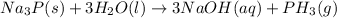
The formula of the gas produced is
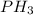 , and the balanced equation is :
, and the balanced equation is :