Answer:
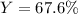
Step-by-step explanation:
Hello there!
In this case, for such stoichiometry problem, we can see just water is on the reactants side, it means that for the calculation of the theoretical yield of oxygen, we use the 2:1 mole ratio between them and their molar masses of 18.02 and 32.00 g/mol respectively:
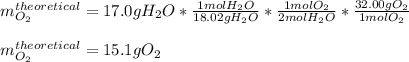
Finally, we divide the actual yield of 10.2 g of oxygen by the theoretical yield of 151 g of oxygen to obtain:
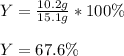
Regards!