Given :
A sample of methane gas (CH₄) with a mass of 58 g is kept in a 1.5 L container at a temperature of 373 K.
To Find :
The pressure of the gas.
Solution :
Moles of methane :
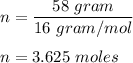
We know, by ideal gas equation :
PV = nRT
Here, R is ideal gas equation,
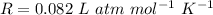
So,

Hence, this is the required solution.