Answer:

Step-by-step explanation:
Molarity is a measure of concentration in moles per liter.
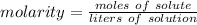
We know the molarity of the solution is 0.99 M and there are 5.3 moles of the solute, CuCl₂.
- 1 molar (M) is equal to 1 mole per liter.
- 0.99 M is equal to 0.99 moles of CuCl₂ per liter.
So,
- molarity= 0.99 mol CuCl₂/L
- moles of solute = 5.3 mol CuCl₂
- liters of solution = x (unknown)
Substitute these values into the formula.
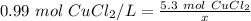
Now, solve for x by isolating the variable. First, cross multiply.
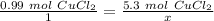
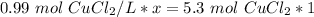
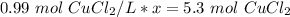
x is being multiplied by 0.99 mol CuCl₂/L. The inverse of multiplication is division. Divide both sides of the equation by 0.99 mol CuCl₂/L .
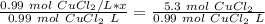
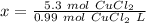
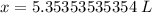
Let's round to the hundredth place. The 3 in the thousandth place tells us to leave the 5 in the hundredth place.
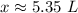
Approximately 5.35 liters are needed to make a 0.99 M solution with 5.3 moles of solute.